Dr. James Fullerton (Supernumerary Fellow, St Hilda’s) uses experimental medicine studies, and in particular human immune challenge paradigms, to better investigate infections, immune responses, and their interaction with drugs. He and his team, consisting of co-investigators Philip Drennan (DPhil student, St Hilda’s), Dr Roel de Maeyer, and Ms Kate Hollett, intentionally give human volunteers substances (e.g. saline solution) that trigger inflammation or activate the immune system in a safe way. This provides a unique opportunity to investigate and understand disease development and immunology in a controlled manner. Findings are more directly translatable to clinical care than when animal models are used, which can help develop new drug therapies more efficiently.
However, the measurements that can be taken in humans to understand how the body reacts to certain challenges are much more limited than in animals. Using a ruler to measure redness, a pen to check swelling, or special imaging tools to see changes in blood flow are easier on participants and do not disturb the body as much. However, they are not as detailed as the more invasive techniques commonly employed in animal research making it more difficult to fully understand the cellular processes underlying the immune response.
Dr Winok Lapidaire [Junior Research Fellow 2020-2023] co-invented the Vascular Imaging Tool for the Auricle (VITA) with Dr. Elliot Bentine. This device produces high resolution images of both the vessels and blood cells in the outer ear. Importantly, it is non-invasive and pain-free for participants. Because of its hand-held and portable design, ease of use, and quick data acquisition (< 1 minute) it can be effortlessly integrated into human immune challenge research setups.
To date, the VITA device has been used to image both microvascular health to study cardiovascular diseases and red blood cells to study anaemia. The original VITA device functions using transmitted light and is specifically tailored to imaging in the ear. However, injections in human immune challenge studies are typically done in the arm, where our existing device would not work. This research project adapted VITA to meet the requirements of these human immune challenge studies, creating a new ‘reflective VITA’ (rVITA) device.
Creating the new rVITA device
Imaging within the arm rather than through the thin tissue of the ear required us to swap from a transmission imaging setup to a reflective imaging setup. After removing the previous backlight illumination, our first task was to design a new sample illumination method to deliver a large amount of light in a small form factor. Sufficient light can be provided by high power LEDs, but these require large heat sinks to avoid temperature rises; geometric constraints prevented these from being placed close to participant skin. We therefore opted for LEDs deep within the device, where ample heat sinking could be provided, and investigated delivery of the light to the sample site through light guides. This would also allow us to bring the light extremely close to the skin to improve illumination in the imaged area. Funds from this grant were used to source optical, electronic and 3D printed components for bench testing these designs.
However, imperfect matching of the LEDs and light guides could lead to large amounts of light lost, and so we modelled various components and optical geometries to determine the best coupling efficiency into the light guides to maximize the available light power. Most standard LEDs at this power level are ideal Lambertian emitters with a 1mm2 emitting area, so we opted for a large 1mm diameter light pipe made from PMMA, which has low losses when guiding light at our wavelengths. After simulating different geometries, we ultimately found that modifying the LED to remove the silicon microlens and then mating the bare LED die to the light pipe was the most efficient in practice.
PMMA is brittle, and when preparing the guides we found that the PMMA would fracture, producing a jagged edge that reduced the amount of light coupled into the guide. We developed a method for heat treating and pressing the PMMA cores to produce an optically flat surface; this increased the transmitted light by a factor of x10.
Redesigning the case
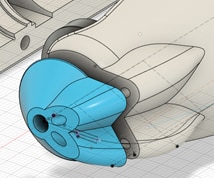
We also had to modify the existing case to use the new reflective imaging mode. First, we removed both the ear clamping mechanism and the optical assembly and electronics for the backlight used in the original transmission imaging. This made space in the device available, which we used to route the light guides from a PCB within the device body to the imaging aperture.
Our goal with the routing was to place the light pipe aperture as close to the imaging area as possible, and we modelled the delivery of light into the tissue. The free variables include the position of the light pipe and the angle of light delivered into the tissue, and the constraints were a need to keep the light guides within the case and minimise their intersection with the imaging system. We also experimented with polishing the light pipe at an angle to improve delivery into the tissue, but found the loss of light was unacceptable.
The front of the device was split into a separate piece so that we could swap out different delivery designs to iterate quickly over which was best. We also increased the magnification of the device and added support for multiple illumination wavelengths, and the device benefitted from other improvements including higher frame rates, and acquisition software and firmware improvements. The final design was printed by Industrial Plastic Fabrications Ltd in a safe, biocompatible PA12 Nylon using funds from the grant.
This collaborative effort, which began at the Green Feast event, brought together the work of two research groups from St Hilda’s College. The multi-disciplinary expertise from immunology, cardiovascular medicine, and physics resulted in the development of a research tool that can facilitate new drug development.